As the air becomes warmer heat is transferred between molecules and kinetic energy is created which produces thermal energy.
Describe the relationship between thermal energy and temperature.
Specific heat and latent heat of fusion and vaporization our mission is to provide a free world class education to anyone anywhere.
And changing the temperature of an object therefore means changing the heat energy it contains.
Explain the meaning of a temperature scale and describe how a particular scale is defined.
Please update your bookmarks accordingly.
Define chemical energy and thermal energy.
The key concept is that heat is a form of energy corresponding to a definite amount of mechanical work.
An example of the way temperature and thermal energy work is how the sun heats air located above the surface of the earth.
Get an answer for describe the relationship between temperature and kinetic energy and find homework help for other science questions at enotes.
The temperature of an object is directly proportional to the amount of heat energy it contains.
We have moved all content for this concept to for better organization.
Cooking a pot of soup c.
Covers heat temperature and thermal energy.
Describe the physical meaning of temperature.
Khan academy is a 501 c 3 nonprofit organization.
As the molecules move faster to transfer heat the temperature also increases.
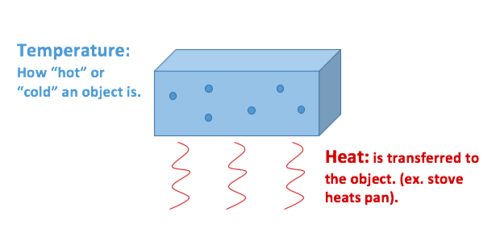
Heat is the transfer of thermal energy between objects that have different temperatures.
Once heat enters a body it becomes its internal energy and no longer exists at heat energy.
Thermal energy always moves from an object with a higher temperature to an object with a lower temperature.
Which best explains the relationship between.
In broad terms thermodynamics deals with the transfer of energy from one place to another and from one form to another.
Convert a temperature expressed in.
Specific heat is the amount of energy in joules.
Watching frost disappear into air.
Most heat energy of the substance is lost.
Melting ice under sunlight d.
Thermodynamics science of the relationship between heat work temperature and energy.
Heat energy is absorbed by the substance.
Which example involves a phase change in which heat energy is released by the substance.
Explain the difference between kinetic energy and potential energy.